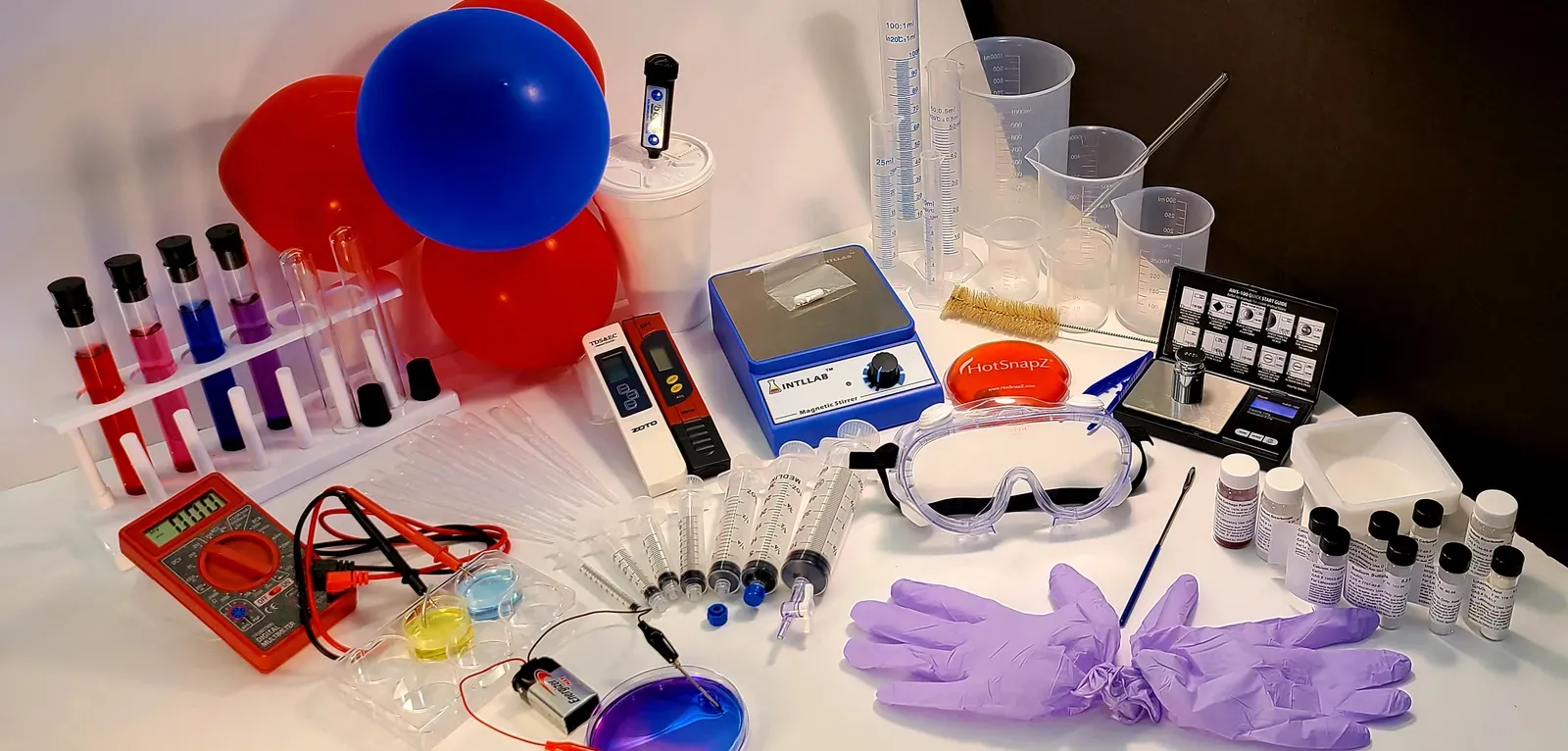
At-home chemistry kits allow students to create lab experiences remotely
Stanford chemistry team delivers hands-on learning to remote students.
When it became clear last spring that in-person classes and labs would no longer be possible due to COVID-19, Jennifer Schwartz Poehlmann, senior lecturer in the Department of Chemistry, started brainstorming. She wanted to explore how to maintain the integrity and rigorous hands-on learning that were part of the introductory undergraduate chemistry sequence she had been teaching in the School of Humanities and Sciences for the last 10 years.
“I believe students have to have hands-on chemistry if they're going to understand it,” Poehlmann said. “When we went into remote mode, students didn't have that opportunity to interact with, observe, and learn from these phenomena firsthand.”
For many students, this sequence is the gateway to future chemistry classes, whether they become chemistry majors, engineers, pre-medical students, or pursue other majors.
In an effort to bring some kind of hands-on experience to summer-quarter remote learners, Poehlmann and lab manager Amanda Nelson spent much of March scouting out available lab kits on the market. But they determined that the pre-packaged kits were not only unaffordable for college students, they did not match their full learning objectives and would have required the chemistry team to change the curriculum.
Laboratory courses regularly include fees to cover materials, and Poehlmann and Nelson hoped to provide a lab kit without substantially increasing costs for students.
“Stanford already has a preeminent laboratory design that has been developed over the last decade by our professors and lecturers as an integral part of the course to make some of the most abstract chemical concepts tangible,” Nelson said. “In short, third-party, hands-on products didn’t match our curriculum and were more expensive.”
So they decided to create their own kits.
Designing the Kits
Creating and mailing a kit that would be affordable and appropriate for at-home experiments turned out to be a huge undertaking. First, Nelson had to identify and locate lab supplies, including plastic beakers and a test tube set that wouldn’t break in the mail.
“Then, there was the Mt. Everest of challenges: safety,” said Nelson. Working closely with Stanford’s office of Environmental Health and Safety (EH&S), Poehlmann and Nelson redesigned the experiments to be educationally engaging but included materials that were safe and stable enough to accommodate a variety of home environments.
Because students might have younger people in their households, the team also planned for the chemicals to be in small quantities, packaged in child-proof containers. They also needed to ensure that the chemicals used in the household “labs” could be safely disposed.
Finally, their proposal was approved. “In the final weeks of spring quarter, we prepared, packed, and shipped 25 kits in a matter of days,” Nelson said.
A Successful Experiment
Students enrolled in the General Chemistry 31A–B sequence this summer have been able to do experiments at home using non-hazardous salts such as calcium chloride and baking soda, Milk of Magnesia, and cabbage powder from their kits. The cabbage powder is a colorful addition, as it changes color based on the reaction occurring.
“It's made from boiled cabbage, and you can concentrate it,” Poehlmann explained. “So anytime a reaction occurs—whether it’s slightly acidic or basic, or even if it's neutral, the cabbage powder indicator changes color. It gives a beautiful visual that helps to illuminate the chemistry that students are observing.”
The entire class, including the laboratory sections, takes place over Zoom, so students are running their experiments at the same time as their peers, with their instructor and teaching assistants virtually present. With a small group participating this summer, the class is taught mid-day Pacific Time, which has worked for the participants.
The teaching assistants, who have been cleared to be in laboratories on campus, demonstrate the more complex or hazardous parts of experiments that might require additional safety precautions such as a laboratory hood for ventilation or special waste disposal.
For instance, to explore periodic trends in metals reactivity, the home kit includes small pieces of calcium and magnesium. But the lab, as originally designed, includes testing lithium, sodium, and potassium as well. These are so highly reactive in pure metal form that they can catch fire when they interact with water, therefore those materials could not be sent to students. Instead students conduct the first two experiments with calcium and magnesium at home and then observe the TA’s remotely as they carry out the reactions with the more reactive metals in the lab.
The students also have their own scale, stir plates, pH and conductivity meters, graduated cylinders for making precise measurements, and balloons, which are used in several different experiments, including one that demonstrates the three-dimensional structures of molecules.
The hard work of bringing the kits to fruition has been rewarded by the feedback of students. “It's been nice to have a more practical, hands-on experience with chemistry in our own homes,” said rising sophomore Amaris Lewis, who is at home in New Orleans, LA. “As I'm doing things like preparing or heating solutions, it helps to be doing these step-by-step processes myself, and understanding why experiments are done in such a specific way really helps concepts from lecture come to life.”
Perhaps the biggest advantage of having undertaken the huge effort of sending the kits to students this summer is the leg up it gives the teaching group in preparing for fall quarter.
During the school year, students in both General Chemistry 31A–B and those in 31M, Chemical Principles: From Molecules to Solids, will benefit from the home kits. And sections will be taught at various times throughout the day to help accommodate students who are in other time zones.
“The course enrollment in the fall is generally 300–400 students,” Poehlmann explained. “One reason we piloted it this summer was with the knowledge that we would almost certainly be teaching students remotely in the fall. A lot of them are going to be freshmen, and we wanted to make sure they had a really great first experience as they're coming into chemistry and the university in general.”




